Virtual Lab Tension Test
ELASTIC DEFORMATION IN MATERIALS
Atomic Basis for Elastic Behavior
Close the window to return to the tension test
Elastic behavior has its origin in the forces between the atoms of the solid, and therefore depends on both the nature of chemical bonding and the crystal structure.
The material properties obtained from the elastic response, namely, E, n , and G, are also known as microstructure – insensitive properties as these quantities are independent of crystalline imperfections, such as interstitial inclusions, vacancies, and dislocations.
The general nature of the atomic bonding and inter-atomic forces also affect the elastic properties of the material. We will rely on that here to qualitatively explain the atomic basis for elastic behavior. When an atomic force is applied along a certain crystal axis in a metal, for example, along a [100] axis, the inter-atomic spacing changes. The spacing increases along the [100] direction and decreases along the two perpendicular directions [010] and [001]. For small extensions, D
r, in the atomic spacing, the force increases linearly with ![]() (see Figure 3 below). The slope of the force vs. inter-atomic distance curve around the equilibrium spacing,
(see Figure 3 below). The slope of the force vs. inter-atomic distance curve around the equilibrium spacing,![]() , is related to the Young’s modulus in the [100] direction. If the extension, D r, is large, the linear relation between force and displacement does not hold.
, is related to the Young’s modulus in the [100] direction. If the extension, D r, is large, the linear relation between force and displacement does not hold.
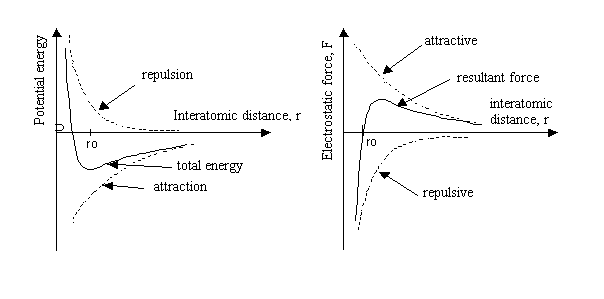
Figure 3. Schematic representation of changes in potential energy as a function of atomic distance
The Young’s modulus can be calculated for materials with different types of atomic bonding.However, the calculation is easiest for materials consisting of ionic bonds, e.g., sodium chloride. The final expression is given below without proof [Ref: Introduction to Mechanical Properties of Materials, MM Eisenstadt, The McMillan Co., New York, 1971, pp. 164- 169]
 (5)
(5)
It should be noted that Young’s modulus is strongly dependent on the quantity ro, and ro depends on the ionic radii of elements forming the solid. The value of E also depends on the product of the magnitude of the cation and anion charges, ![]() and
and ![]() , respectively. Also e is the charge in an electron, n is the exponent in the repulsive potential term, and n
is the Poisson’s ratio. The shear modulus, G, and the Young’s modulus, E, both depend on ro in the same way, as in equation (5), because they are related through equation (3). Relations similar to equation (5) can be derived for metallic and covalent bonded materials, but the derivation is more complicated and beyond the scope of this article.
, respectively. Also e is the charge in an electron, n is the exponent in the repulsive potential term, and n
is the Poisson’s ratio. The shear modulus, G, and the Young’s modulus, E, both depend on ro in the same way, as in equation (5), because they are related through equation (3). Relations similar to equation (5) can be derived for metallic and covalent bonded materials, but the derivation is more complicated and beyond the scope of this article.
The concept of the elastic modulus can also be qualitatively perceived as being the ‘spring stiffness’ at an atomic level. In the spring model, inter atomic forces are applied to the atoms through extensions or contractions of springs between atoms. If all the atoms are in the equilibrium position, ro, the spring force is zero. If an atom is displaced through a small distance, ![]() = r - ro, the force exerted on a spring is F = k
= r - ro, the force exerted on a spring is F = k![]() , where k is the spring stiffness. The value of k is in the range 20-200 N/m for covalent bonds, and 15-100 N/m for metallic and ionic bonds. Hence covalent bonded solids are stiffer. In polymers the chains consist of atoms held together by covalent bonds, but the inter-chain bonding is of the weak Van der Waals type, resulting in a low spring constant of 0.5-2 N/m. Hence long chain polymers have low elastic modulus.
, where k is the spring stiffness. The value of k is in the range 20-200 N/m for covalent bonds, and 15-100 N/m for metallic and ionic bonds. Hence covalent bonded solids are stiffer. In polymers the chains consist of atoms held together by covalent bonds, but the inter-chain bonding is of the weak Van der Waals type, resulting in a low spring constant of 0.5-2 N/m. Hence long chain polymers have low elastic modulus.
Engineering Quantities Obtained from a Tension Test
![]()
where l = final length
lo = original length
![]()
where P = applied load
Ao = original area of cross-section of the tensile specimen over the gage length
![]()
Over a small range of strain (less than 0.2% strain for metals), the material generally deforms elastically. If the load is removed, the material of the specimen returns to its original length. This is called the linear elastic behavior. The modulus of elasticity is defined only in the linear elastic range. E is this the slope of the s-e curve in the elastic range.
The elongation of the specimen is accompanied by a concentration of its lateral dimensions. The absolute value of the ratio of the lateral strain to longitudinal strain is known as the Poisson’s ratio.
![]()
Note: A separate strain gage has to be mounted on the specimen to measure the lateral strain.
This is the area under the stress-strain curve up to the yield point Sy of the material. It is a measure of the energy the material can store elastically.
Modulus of Resilience = ![]() =
= ![]()
where Sy = yield strength of material
![]() = strain at the yield point
= strain at the yield point
Note: units of modulus of resilience are energy per unit volume, e.g. , N-m / m3 or lb-in / in3